
Achieving Premium Brine Quality in Chlor-Alkali Production through Chelating Resins
The quality of brine in chlor-alkali production is crucial. Traditional primary brine purification does not meet the required standards, so secondary purification of the brine is necessary. In the secondary brine purification process, ion exchange resins are generally used to remove Ca²⁺, Mg²⁺, and heavy metals. These devices utilize the selectivity and equilibrium reaction principles of anion and cation exchange resins to remove electrolyte ions from water. This process is essential for producing high-purity water. After the brine is refined through the ion exchange filter, the secondary brine can achieve a total mass fraction of Ca²⁺ and Mg²⁺ of less than 20 ppb.
I. Technical Background
In chlor-alkali production, the quality of brine not only affects the power consumption of caustic soda products but also impacts the lifespan of the electrolysis membranes used in electrolytic cells. Enhancing the quality of brine has always been a key technical challenge. Ion-exchange electrolytic cells have very high requirements for brine quality. Improving the quality of refined brine is critical for ensuring stable production and increasing economic benefits.
To prevent the deterioration of ion-exchange membrane performance, it is necessary to control the total mass fraction of Ca²⁺ and Mg²⁺ to below 20 ppb. Additionally, corresponding requirements exist for the mass fractions of Sr²⁺ and Ba²⁺. Traditional primary brine treatment methods cannot meet these stringent requirements, necessitating secondary brine purification.
II. Application of Chelating Resin in Secondary Brine Purification
Chelating resins are cross-linked functional polymer materials that can form chelating complexes with metal ions. Compared to ion exchange resins, chelating resins have stronger binding forces and higher selectivity. They are widely used in the recovery and separation of various metal ions, amino acid separation, hydrometallurgy, pollution control, and other fields.
Ion-exchange membrane caustic soda production has higher purity requirements for secondary brine. Traditional precipitation processes struggle to reduce harmful ions in brine to acceptable levels. The aminophosphonic acid resins and aminodiaceticc acid resins developed by Sunresin can effectively remove ions such as Ca²⁺, Mg²⁺, Sr²⁺, Ba²⁺, Fe²⁺, Ni²⁺, and Al³⁺ from the brine. After primary brine is treated with Sunresin's chelating resins, the levels of Ca²⁺ and Mg²⁺ in the secondary brine can be controlled to much lower than the current requirement of 20 ppb, fully meeting the requirements of the ion-exchange membrane process. This improves current efficiency and reduces the damage caused by harmful metal ions to the electrolytic cells and ion-exchange membranes.
III. Selection of Sunresin's Chelating Resins for Chlor-alkali Industry: SEPLITE® LSC710
Sunresin's SEPLITE® LSC710 chelating resin is a weakly acidic macroporous chelating resin of the iminodiacetic acid type, used for the selective removal of alkaline earth metals and heavy metal cations. In the ion-exchange membrane caustic soda industry, it refines and purifies secondary brine, forming stable chelates with divalent metal ions. Compared to aminophosphonic acid chelating resins, the iminodiacetic acid chelating resin is more effective in removing heavy metals such as strontium.
Working Principle:
In the ion-exchange membrane process for caustic soda production, the typical reactions for removing cations from brine are:
Service Cycle:
RCH₂N(CH₂COONa)₂ + Ca²⁺ → RCH₂N(CH₂COO)₂Ca + 2Na⁺
Regeneration:
RCH₂N(CH₂COO)₂Ca + 2HCl → RCH₂N(CH₂COOH)₂ + CaCl₂
Conditioning:
RCH₂N(CH₂COOH)₂ + 2NaOH → RCH₂N(CH₂COONa)₂ + 2H₂O
In these reactions, the chelating resin RCH₂N(CH₂COOH)₂ reacts with divalent metal ions such as Ca²⁺, Mg²⁺, and Sr²⁺, forming stable chelate complexes RCH₂N(CH₂COO)₂Ca and releasing hydrogen ions (H⁺) or sodium ions (Na+)in case of resin operated in sodium form. This process effectively removes these hardness ions from the brine, thus purifying it to meet the stringent quality requirements for ion-exchange membrane caustic soda production.
Selectivity Order:
- Under Acidic Conditions: Pb2+ > Cu2+ > U4+ > Zn2+ > Al3+ > Mg2+ > Sr2+ > Ca2+ > Na+ > Ba2+
- Under Alkaline Conditions: Mg2+ > Ca2+ > Sr2+ > Al3+ > Ba2+ ≥ Na+ > K+
SEPLITE® LSC750 Chelating Resin
SEPLITE® LSC750 chelating resin is a macroporous, uniformly sized resin with weakly acidic aminophosphonic acid active groups, cross-linked with styrene and divinylbenzene. This chemical structure facilitates the formation of chelates with metal ions. Compared to iminodiacetic acid resins, aminophosphonic acid chelating resins exhibit a higher affinity for divalent cations and form more stable chelates with low atomic weight and low-valence cations. Therefore, SEPLITE® LSC750 aminophosphonic acid chelating resin is more suitable for secondary brine purification in ion-exchange membrane caustic soda production, whereas iminodiacetic acid type chelating resins are better suited for removing heavy metal ions.
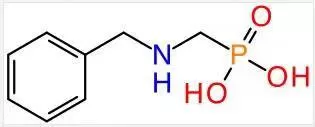
SEPLITE® LSC750 Resin Structural Formula
Working Principle:
In the ion-exchange membrane process for caustic soda production, the typical reactions for removing hard ions from brine are:
Service Cycle:
RCH₂NHCH₂PO₃Na₂ + Ca²⁺ → RCH₂NHCH₂PO₃Ca + 2Na⁺
Regeneration:
RCH₂NHCH₂PO₃Ca + 2HCl → RCH₂NHCH₂PO₃H₂ + CaCl₂
Conditioning:
RCH₂NHCH₂PO₃H₂ + 2NaOH → RCH₂NHCH₂PO₃Na₂ + 2H₂O
Selectivity Order:
- Under Acidic Conditions (pH acidic): Pb2+ > Cu2+ > U4+ > Zn2+ > Al3+ > Mg2+ > Sr2+ > Ca2+ > Na+ > Ba2+
- Under Alkaline Conditions (pH basic): Mg2+ > Ca2+ > Sr2+ > Al3+ > Ba2+ ≥ Na+ > K+
IV. Application of Uniform Particle Size Chelating Resins
To adapt to developments in the ion-exchange membrane caustic soda industry, Sunresin has developed SEPLITE® LSC750 (aminomethylphosphonic acid) and SEPLITE® LSC710 (iminodiacetic acid) chelating resins. Building on this foundation, Sunresin has obtained a full set of patents for the production equipment of uniform particle size chelating resins. Sunresin has introduced the improved SEPLITE® Monojet™ LSC7100 iminodiacetic acid and SEPLITE® Monojet™ LSC7500 aminomethylphosphonic acid uniform particle size chelating resins.
Sunresin is currently the only company in China with the capability to produce uniform particle size chelating resins, filling the domestic gap in this area. These advancements effectively enhance the efficiency and extend the lifespan of ion-exchange membranes.
SEPLITE® Monojet™ LSC7100 and SEPLITE® Monojet™ LSC7500 Chelating Resins
SEPLITE® Monojet™ LSC7100 and SEPLITE® Monojet™ LSC7500 are macroporous, uniform particle size chelating resins developed and produced by Sunresin. These resins have a styrene backbone and feature iminodiacetic acid and aminophosphonic acid groups, respectively. Their chemical structures are conducive to forming chelates with metal ions, enabling selective adsorption.
Both iminodiacetic acid chelating resins and aminophosphonic acid chelating resins exhibit high affinity for metal cations and easily form stable chelates with divalent metal cations. Consequently, SEPLITE® Monojet™ LSC7100 and SEPLITE® Monojet™ LSC7500 uniform particle size chelating resins are suitable for secondary brine purification in the ion-exchange membrane process for caustic soda production.
Advantages of SEPLITE® Monojet™ LSC7100 and SEPLITE® Monojet™ LSC7500 Uniform Particle Size Chelating Resins
- Faster Exchange Speed and Higher Working Exchange Capacity: These resins demonstrate more efficient ion exchange processes and can handle larger volumes of metal ions.
- Better Hydraulic Characteristics During Operation: Improved flow dynamics result in lower system pressure drops during operation.
- Significant Improvement in Brine Treatment Volume and Efficiency: They can process greater quantities of brine more efficiently in each cycle.
- Higher Resistance to Osmotic Shock: Lower breakage rates under similar operating conditions, reducing the need for resin replenishment and replacement
- Longer Lifespan with Advanced Granulation Technology: Manufactured using internationally leading jet granulation technology, these resins offer enhanced durability.
- Excellent Uniformity and Larger Effective Void Volume: The resins' uniform particle size provides greater effective porosity, improving overall performance.
V. Application Cases
Sunresin's chelating resins for ion-exchange membrane caustic soda production have been applied in various brine preparation processes using refined salt, mineral salt, refined salt, brine, sea salt, and composite salts. These resins are suitable for refining brine after primary purification using different types of membranes such as Kaimem, Gomem, planting membranes, and ceramic membranes. The resulting brine indices are stable, and the secondary brine indices are superior to similar chelating resins from domestic and international sources.
Sunresin's chelating resins hold the leading market share among ion-exchange membrane caustic soda clients. These resins have been successfully implemented in over 120 ion-exchange membrane enterprises domestically and internationally. They are used in complete line supplies for electrolytic cells from suppliers like Xu Hua Cheng, Chlor Engineering, Wood, and North Chemical Machinery, meeting all the requirements of various brine purification units. This has significantly reduced costs for customers and gained high recognition from them.
In the chlor-alkali industry, the quality of brine is paramount to the efficiency and longevity of production processes. Chelating resins, such as those developed by Sunresin, offer superior removal of harmful ions like Ca²⁺ and Mg²⁺, ensuring the brine meets the stringent requirements necessary for optimal ion-exchange membrane performance. With advancements like the SEPLITE® LSC710, SEPLITE® LSC750, SEPLITE® Monojet™ LSC7100, and SEPLITE® Monojet™ LSC7500 resins, Sunresin has set a new standard in brine purification, significantly improving operational efficiency and reducing costs for chlor-alkali producers.
Ready to enhance the quality and efficiency of your chlor-alkali production process? Discover the advanced capabilities of Sunresin's chelating resins today. Contact our team to learn more about how our innovative solutions can meet your specific needs and drive your business forward. Reach out now to experience unparalleled brine purification and take your production to the next level.
Free Quote
Resources
Adsorbent Resin
Bio-Pharmaceutical & Life Science
Enzyme Carriers
Hydrometallurgy & Mining
Chelating Resin
Chemical Industry
Chromatographic Media
Wastewater Treatment&Reuse
Food & Beverage Industries
Ion Exchange Resin
Civil & Industry Water Treatment
Equipments And Projects
Plant Extraction
Catalyst Resin
Solid Phase Peptide Synthesis
Product
Application
Contact Us
Sunresin Park,No.135, jinye Road, Xi’an Hi-tech Industrial Development Zone, Shaanxi-710076, China
seplite@sunresin.com
seplite_europe@sunresin.com
+86-29-89182091
Our Product List
Latest News
30
2024 08
Sunresin Makes Hurun China Top 500 List
The Hurun Research Institute recently released their "2023 Hurun China Top 500" list, with Sunresin has making the list in recognition of its outstanding market performance and innovation capabilities. This is the only company on the list in China's adsorption separation materials industry.
22
2024 06
Exhibition Highlights | Sunresin Showcases at SIWW 2024 Singapore International Water Week
From June 19-21, 2024, the Singapore International Water Week (SIWW Water Expo 2024) was held at the Marina Bay Sands Expo and Convention Centre in Singapore. Sunresin showcased its internationally leading products and technologies at the event.
10
2024 05
Sunresin and Latin America: Ten thousand miles are still neighbors
In April, Ambassador Xu Yicong's book launch of "Family and Country Sentiments - Continuing Chapter" was successfully held in Beijing. The ambassadors of Latin America in China have come to congratulate Ambassador Xu, and Zhai Feng, Vice President of Bump Cycle, attended to celebrate the event. Dr. Gao Yuejing, as the representative of the important guests, gave a speech and expressed her most sincere blessing to Ambassador Xu for the publication of his new book.
Leave a Message
Please send any questions you want to know, we will reply to you immediately.
Choose File
Submit















