
Arsenic Poisoning: Removal of Arsenic Contamination in Drinking Water Using Ion Exchange Resin
Chemical Properties and Hazards of Arsenic
Arsenic is a metalloid element, commonly known as arsenic trioxide, is a highly toxic substance. On July 23, 2019, arsenic and its compounds were included in the list of toxic and hazardous water pollutants (first batch).
The arsenic content in surface water is relatively safe, with very few surface water sources exceeding 5 μg/L. However, arsenic levels in groundwater can reach higher levels. Water treatment plants that use groundwater sources for drinking water must adopt appropriate treatment processes to remove excessive arsenic from the water. According to the "Standards for Drinking Water Quality" (GB5749-2006), the arsenic content in drinking water should be less than 0.01 mg/L.
Causes and Hazards of Excess Arsenic in Groundwater
Both natural groundwater and surface water may contain arsenic, with groundwater typically having higher arsenic concentrations than surface water. The arsenic content in surface water is relatively safe, with very few sources exceeding 5 μg/L. However, groundwater can have much higher levels of arsenic. Water plants that use groundwater as a source for drinking water must adopt appropriate treatment processes to remove excessive arsenic. According to the "Standards for Drinking Water Quality" (GB5749-2006), the arsenic content in drinking water should be less than 0.01 mg/L.
Water Quality Standards and Limits
| Indicator | Limit Value |
|---|---|
| Microbiological Indicators | |
|
Total coliform bacteria (MPN/100mL or CFU/100mL) |
Not detectable |
|
Thermotolerant coliform bacteria (MPN/100mL or CFU/100mL) |
Not detectable |
|
Fecal coliform bacteria (MPN/100mL or CFU/100mL) |
Not detectable |
| Total bacterial count (CFU/mL) | 100 |
| Toxicological Indicators | |
| Arsenic (mg/L) | 0.01 |
| Cadmium (mg/L) | 0.005 |
| Chromium (Hexavalent) (mg/L) | 0.05 |
| Lead (mg/L) | 0.01 |
| Mercury (mg/L) | 0.001 |
| Selenium (mg/L) | 0.01 |
| Cyanide (mg/L) | 0.05 |
| Fluoride (mg/L) | 1.0 |
| Nitrate (as Nitrogen, mg/L) | 10 (20 for groundwater sources) |
| Other Chemical Indicators | |
| Trichloromethane (mg/L) | 0.06 |
| Tetrachloromethane (mg/L) | 0.002 |
| Chloramines (as Cl2, mg/L) | 0.01 |
| Chlorophenol (as Cl2, mg/L) | 0.9 |
|
Chlorite (when using chlorine dioxide for disinfection, mg/L) |
0.7 |
|
Chlorate (when using combined chlorine dioxide disinfection, mg/L) |
0.7 |
Table: Groundwater Quality Indicators
Causes and Hazards of Excess Arsenic in Groundwater
Industrial Sources
Industrial production is a significant source of arsenic contamination, particularly in non-ferrous metallurgy and mining. Arsenic-bearing minerals such as arsenopyrite, realgar, orpiment, and enargite are commonly found alongside other non-ferrous metal minerals. Due to natural release and human activities, especially the extensive mining and use of low-grade ores, large amounts of arsenic-containing waste and by-products are generated in the mining and smelting of non-ferrous metals. These include arsenic-laden dust from pyrometallurgy and purification residues from hydrometallurgy. Additionally, wastewater from sulfuric acid preparation, chemical dye and pesticide production, wood processing, and the glass and ceramics industries often contains high concentrations of arsenic.
Health Impacts
Arsenic can enter the human body through the respiratory tract, food, or skin contact, accumulating in organs or tissues such as the liver, kidneys, bones, and hair. It can damage the digestive and nervous systems. Long-term consumption of high-arsenic water can lead to skin diseases such as keratosis and pigmentation changes, blackfoot disease, neurological disorders, vascular damage, and an increased risk of heart disease. In regions like Inner Mongolia, Xinjiang, and Taiwan in China, arsenic levels in drinking water have been recorded at 0.2-2.0 mg/L, significantly exceeding the national drinking water standard of less than 0.05 mg/L. This has led to endemic arsenic poisoning. Removing arsenic from drinking water is a critical measure to prevent and control endemic arsenic poisoning.
Arsenic Removal Methods
Current arsenic removal methods reported domestically and internationally include:
- Biological methods
- Coagulation methods
- Precipitation methods
- Adsorption methods
- Ion exchange methods
These methods are implemented to effectively reduce arsenic levels in drinking water and mitigate the associated health risks.
Groundwater and Drinking Water Arsenic Removal Processes
Biological Methods
The principle of biological methods involves special bacterial strains that produce substances similar to activated sludge during their cultivation. These substances use flocculation to bind with arsenic, forming a precipitate, thereby achieving arsenic removal. However, the cultivation cycle for these bacterial strains is long and requires stringent environmental conditions. Thus, biological methods are generally used for arsenic removal in wastewater, with rare applications reported for drinking water.
Coagulation Methods
The principle of coagulation methods is to use coagulants with strong adsorption capabilities to adsorb arsenic, converting it into a precipitate. This precipitate is then separated from the water through filtration and other means. Coagulation methods are cost-effective, easy to operate, and highly efficient at arsenic removal. They can effectively treat industrial wastewater to meet discharge standards and ensure drinking water meets safe standards. Therefore, coagulation is widely used in industrial production and the treatment of drinking water. However, coagulation methods require large amounts of coagulants, generate significant amounts of arsenic-containing waste sludge that is difficult to handle, and if accumulated over time, can lead to secondary pollution, limiting the method's application.
Precipitation Methods
The principle of precipitation methods is to use chemical reactions to convert arsenic into a precipitate, which is then removed through filtration. Precipitation methods have clear advantages for the preliminary treatment of industrial high-arsenic wastewater but are not suitable for treating trace amounts of arsenic in drinking water. Therefore, treated arsenic-containing wastewater usually requires additional methods for deep treatment to meet discharge standards.
Adsorption Methods
The principle of adsorption methods involves using solid materials with high specific surface areas and insolubility as adsorbents. These adsorbents use mechanisms such as physical adsorption, chemical adsorption, or ion exchange to fix arsenic contaminants from water onto their surfaces, achieving arsenic removal. The effectiveness of adsorption methods can be influenced by organic matter, pH levels, the form and concentration of arsenic in water, and the presence and concentration of other ions. Additionally, adsorbent materials are often expensive. Pre-treatment measures, such as multi-stage filtration before using the adsorbents, can be employed to enhance the efficiency of adsorption methods.
Ion Exchange Method
The principle of the ion exchange method involves using anion exchange resins with exchangeable ions that react with arsenic ions in water to remove arsenic. This method produces only 20% of the sludge volume compared to chemical precipitation methods, significantly reducing sludge disposal costs. Additionally, the ion exchange method has a large treatment capacity, is simple to operate, easy to regenerate, and has good separation efficiency. It can meet stringent discharge standards and facilitate the recovery of various valuable components. Therefore, it is considered a promising method for arsenic removal and is increasingly being applied in various arsenic removal scenarios.
These methods are implemented to ensure the safe removal of arsenic from groundwater and drinking water, preventing health hazards associated with arsenic contamination.
Summary of Arsenic Removal Methods
| Method | Principle | Advantages | Disadvantages | Applications |
|---|---|---|---|---|
| Biological |
Special bacteria produce substances that bind with arsenic to form precipitates. |
Effective for wastewater arsenic removal. |
Long cultivation cycle, stringent environmental requirements. |
Wastewater treatment |
| Coagulation |
Coagulants adsorb arsenic, forming a precipitate that is then filtered out. |
Cost-effective, easy to operate, high efficiency. |
Requires large amounts of coagulants, produces significant waste sludge, potential for secondary pollution. |
Industrial and drinking water |
| Precipitation |
Chemical reactions convert arsenic into a precipitate that is filtered out. |
Effective for high- arsenic industrial wastewater. |
Not suitable for trace arsenic in drinking water. |
Industrial wastewater treatment |
| Adsorption |
Solid materials with high surface area adsorb arsenic through physical or chemical mechanisms. |
Effective for various arsenic concentrations, adaptable to pre- treatment measures. |
Influenced by organic matter, pH, and other ions, expensive adsorbent materials. |
Drinking and wastewater treatment |
|
Ion Exchange |
Anion exchange resins exchange ions with arsenic ions in water. |
Low sludge production, cost-effective, large treatment capacity, easy to operate, good separation. |
Requires regeneration of resins, potential high initial cost. |
Broad applications |
The ion exchange method stands out for its efficiency, low operational costs, and ability to meet strict environmental standards, making it a versatile and increasingly popular choice for arsenic removal in both drinking water and industrial applications.
Arsenic Removal Process Using Ion Exchange
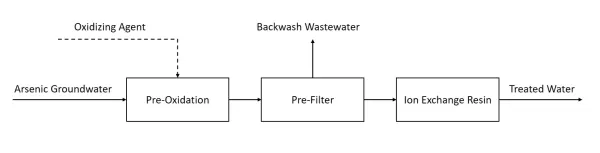
Figure: Ion exchange process for arsenic removal
1.Oxidation:
- Oxidizing Agent: An oxidizing agent is added to the arsenic-containing groundwater.
- Pre-oxidation: This step converts arsenic (III) to arsenic (V), which is more easily removed by ion exchange resins.
2.Pre-filtration:
- The water undergoes a pre-filtration step to remove any particulate matter that could clog the ion exchange resin.
3.Ion Exchange Resin:
- Ion Exchange: The pre-filtered water passes through an ion exchange resin, where arsenic ions are exchanged with other ions on the resin, effectively removing the arsenic from the water.
4.Backwash Wastewater:
- The ion exchange system is periodically backwashed to remove accumulated contaminants from the resin. The backwash water is treated as wastewater.
5.Treated Water:
- The treated water, now free of arsenic, is collected as output water ready for use.
Sunresin´s Ion Exchange Arsenic Removal Process
Using Sunresin's ion exchange arsenic removal process as an example, the SEPLITE® LAR714 arsenic removal resin, which selectively removes certain ions, is employed. This resin is utilized for its high adsorption capacity and adsorption rate, effectively removing arsenic compounds from drinking water and groundwater.

SEPLITE® LAR714 arsenic removal resin is a type of polymer resin with functional groups, boasting excellent physical and chemical properties. It has superior strength indicators, meeting the requirements for higher resin bed heights. It is used to remove arsenic from water, food, sugar solutions, and other liquids. The resin can adsorb up to 1.4g of arsenic per liter of resin, with a removal precision of up to 5ppb or less (the adsorption capacity and removal precision depend on the arsenic content in the feed solution).
To enhance the selectivity of the resin for arsenic, BlueStar Sunresin incorporates functional groups with specific adsorption functions into the resin framework. This results in exceptionally high selectivity, achieving directional adsorption of arsenic at a rate of over 95%. Additionally, through special processes, the functional groups are ensured to have exceptional robustness and durability.
Advantages of the SEPLITE® LAR714 Resin
Currently, the SEPLITE® LAR714 Resin has achieved industry recognition in the chemical, food, and drinking water sectors, offering the following advantages:
- Selective Adsorption: The special structure of the resin's functional groups is selectively adsorptive towards arsenates and arsenites.
- Stable and Efficient: The treated water can have arsenic levels reduced to below 5 ppb, with no arsenic detected above the limit during operation. The adsorption efficiency is typically over 99%.
- Economic and Reliable: The resin maintains stable exchange capacity after saturation and regeneration, allowing for reuse. The operational energy consumption of the equipment is low.
- Easy Operation and Maintenance: The system is highly automated, with options for automatic or manual control of the water flow and regeneration process.
- Various Equipment Specifications: Equipment can be designed in different specifications based on the water volume, easily handling different water quantities.
- Space-Saving: The equipment has a small footprint, conserving space.
These features make the SEPLITE® LAR714 resin a versatile and practical solution for arsenic removal across multiple industries.
SEPLITE® LAR714 Resin Arsenic Removal Case Study
Drinking Water Arsenic Removal Case
A drinking water company in Guangxi implemented an arsenic removal project using SEPLITE® LAR714 resin. The source water was tap water with an arsenic content of approximately 80 ppb. The system was designed to operate with a flow rate of 12.5 m³/h and run continuously for 24 hours. Based on the project's specific conditions, business requirements, and relevant standards, Sunresin employed an ion exchange arsenic removal system. The treated water had an arsenic content reduced to below 10 ppb. The resin used in the system is capable of being regenerated and reused.
This case highlights the effectiveness and efficiency of the SEPLITE® LAR714 resin in removing arsenic from drinking water, ensuring compliance with safety standards and providing a reliable solution for continuous operation.
Arsenic Removal Case in the Semiconductor Industry
In a special arsenic removal project at a semiconductor production facility, gallium arsenide (GaAs) was used in the manufacturing process, resulting in arsenic-containing wastewater that needed treatment. The wastewater had an arsenic concentration of 11.287 ppm, a pH of 7.69, was a colorless and transparent liquid, and had a conductivity of 464 µS/cm. Blue Sky Technology used an arsenic removal ion exchange resin process, reducing the arsenic concentration in the effluent to below 0.3 ppm, meeting the client's requirements and satisfying the Class III water quality standards as specified in the "Surface Water Environmental Quality Standards." Additionally, the treated water was stable.
Additions not contained in the original document:
Summary
The SEPLITE® LAR714 resin by Sunresin has demonstrated exceptional performance in arsenic removal from various water sources such as drinking water, achieving industry recognition for its high efficiency, stability, and economic viability. The resin's selective adsorption properties, combined with its ability to be regenerated, make it a reliable choice for continuous operation in various applications.
Sunresin`s Dedication
Sunresin is committed to addressing environmental challenges through innovative solutions. Our dedication to driving innovation in water treatment technologies is evident in the development of the SEPLITE® LAR714 resin. By leveraging advanced research and development, Sunresin aims to provide effective, sustainable, and economical solutions to ensure safe drinking water and protect public health.
Join us in our mission to ensure clean and safe drinking water for all. Contact Sunresin today to learn more about our innovative arsenic removal solutions and how we can help you meet your water treatment needs. Together, we can make a significant impact on public health and the environment. Reach out to our team for consultation and take the first step towards a cleaner, safer future.
Free Quote
Resources
Adsorbent Resin
Bio-Pharmaceutical & Life Science
Enzyme Carriers
Hydrometallurgy & Mining
Chelating Resin
Chemical Industry
Chromatographic Media
Wastewater Treatment&Reuse
Food & Beverage Industries
Ion Exchange Resin
Civil & Industry Water Treatment
Equipments And Projects
Plant Extraction
Catalyst Resin
Solid Phase Peptide Synthesis
Product
Application
Contact Us
Sunresin Park,No.135, jinye Road, Xi’an Hi-tech Industrial Development Zone, Shaanxi-710076, China
seplite@sunresin.com
seplite_europe@sunresin.com
+86-29-89182091
Our Product List
Latest News
30
2024 08
Sunresin Makes Hurun China Top 500 List
The Hurun Research Institute recently released their "2023 Hurun China Top 500" list, with Sunresin has making the list in recognition of its outstanding market performance and innovation capabilities. This is the only company on the list in China's adsorption separation materials industry.
22
2024 06
Exhibition Highlights | Sunresin Showcases at SIWW 2024 Singapore International Water Week
From June 19-21, 2024, the Singapore International Water Week (SIWW Water Expo 2024) was held at the Marina Bay Sands Expo and Convention Centre in Singapore. Sunresin showcased its internationally leading products and technologies at the event.
10
2024 05
Sunresin and Latin America: Ten thousand miles are still neighbors
In April, Ambassador Xu Yicong's book launch of "Family and Country Sentiments - Continuing Chapter" was successfully held in Beijing. The ambassadors of Latin America in China have come to congratulate Ambassador Xu, and Zhai Feng, Vice President of Bump Cycle, attended to celebrate the event. Dr. Gao Yuejing, as the representative of the important guests, gave a speech and expressed her most sincere blessing to Ambassador Xu for the publication of his new book.
Leave a Message
Please send any questions you want to know, we will reply to you immediately.
Choose File
Submit














