
SEPLIFE®, All You Need to Know about Ion Exchange Chromatography
Introduction to Ion Exchange Chromatography:
Ion exchange chromatography is a column chromatography method that uses the difference in electrostatic force between the exchangeable ions on the ion exchanger and the various ions separated in the surrounding medium, and achieves the purpose of separation through exchange equilibrium. Ion-exchange chromatography has the advantages of high sensitivity, repeatability, good selectivity, and fast analysis speed, and is currently one of the most commonly used chromatography methods.
In 1848, Thompson et al. discovered the phenomenon of ion exchange in the process of studying the exchange of alkaline substances in soil. In the 1940s, polystyrene ion exchange resins with stable exchange characteristics appeared. In the 1950s, ion exchange chromatography entered the field of biochemistry and was applied to the analysis of amino acids. At present, ion exchange chromatography is still a commonly used chromatography method in the field of biochemistry, and is widely used in the separation and purification of various biochemical substances such as amino acids, proteins, sugars, viruses, and nucleotides.
The Ion Exchange process:
The reaction between the ion exchanger and the ions or ionic compounds in the solution is mainly carried out by ion exchange. The ion exchange reaction performed is reversible. Assuming that RA represents the cation exchanger, the cation A+ dissociated in the solution can undergo a reversible exchange reaction with the cation B+ in the solution, and the reaction formula is:
RA + B+ → RB + A+
The reaction reaches equilibrium at an extremely rapid rate, and the equilibrium shift follows the law of mass action.
The selectivity of an ion exchanger can be expressed by the equilibrium constant K of its reaction:
K=[RB][A+]/[RA][B+]
✔If [A+] is equal to [B+] in the reaction solution, then K=[RB]/[RA].
✔If K>1, that is, [RB]>[RA], it means that the binding force of the ion exchanger to B+ is greater than that of A+;
✔If K=1, that is, [RB]=[RA], it means that the ion exchanger has the same binding force to A+ and B+;
✔If K<1, that is, [RB]<[RA], it means that the binding force of the ion exchanger to B+ is less than that of A+.
✔The K value is a parameter that reflects the binding force or selectivity of the ion exchanger to different ions, so the K value is called the selectivity coefficient of the ion exchanger for A+ and B+.
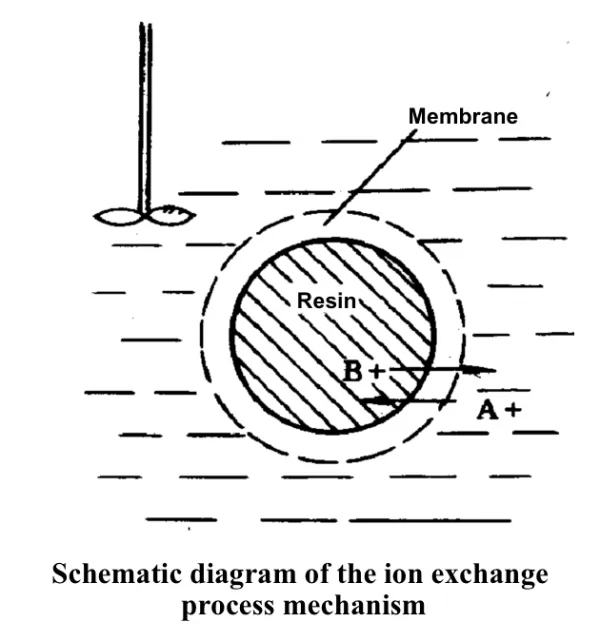
Mechanism of ion exchange:
A+ diffuses from the solution to the resin surface.
A+ enters the active center inside the resin from the surface of the resin.
A+ undergoes metathesis reaction with RB on the active center.
The desorbed ion B+ diffuses from the interior of the resin to the surface of the resin.
B+ ions diffuse from the resin surface into the solution.
The controlling step of the exchange rate is the rate of diffusion, which may be controlled by internal diffusion or external diffusion in different separation systems.
Factors Affecting the Ion Exchange Process:
✔Particle size: the smaller, the faster
✔Degree of cross-linking: small degree of cross-linking, fast exchange speed
✔Temperature: the higher the faster, it is related to the increase of the diffusion coefficient
✔Ion valence: the higher the valence, the slower the diffusion rate
✔Ion size: the smaller the faster
✔ Stirring speed: to a certain extent, the bigger the faster
✔Solution concentration: When the exchange rate is controlled by diffusion, the greater the concentration, the faster the exchange rate
Principle of ion exchange:
If a cation exchange resin is selected, the positively charged substance is exchanged with H+ and bound to the resin. If an anion exchange resin is selected, the negatively charged substance can be exchanged with OH- and bound to the resin.
There are differences in the degree of firmness of the combination of substances on the resin, and the components in the mixture can be eluted one by one by selecting an appropriate eluent to achieve the purpose of separation and purification.
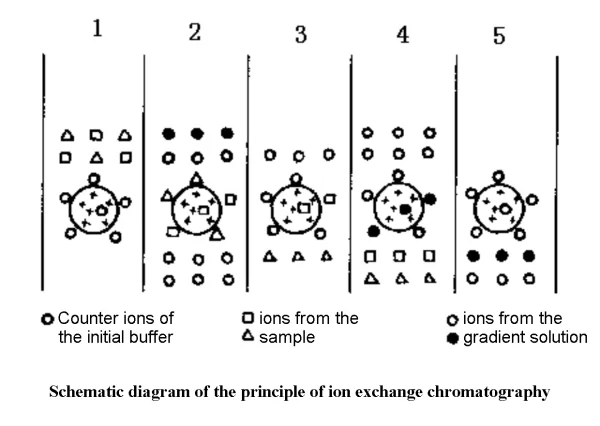
✔1. Equilibration stage: combination of ion exchanger and counter ion
✔2. Adsorption stage: sample and counter ion exchange
✔3. Desorption stage: the gradient buffer solution first washes off the weakly adsorbed substances, and then washes off the strongly adsorbed substances
✔4. Regeneration stage: fully wash with the original balance solution, which can be reused
Ion exchange chromatography resins:
The charged groups of cation exchangers are negatively charged, and the counter ions are positively charged, which can perform exchange reactions with cations or positively charged compounds in solution.
According to the strength of the charged group, it can be divided into three types, respectively strong acid type (group with sulfonic acid, R-SO3H), medium-strong acid type (containing phosphoric acid group or phosphorous acid group, R-PO3H2) and weak acid type (with carboxyl group and phenol-based resin, R-COOH or R-benzene ring-OH).
During the exchange of these exchangers, the hydrogen ions are replaced by foreign cations, as shown in the following formula:
R-COOH+Na+=R-COONa++H+
The anion exchanger is formed by introducing quaternary amine [-N (CH3) 3], tertiary amine [-N (CH3) 2], secondary amine [-NHCH3] and primary amine [-NH2] groups on the matrix.
According to the different level of alkalinity of the amine groups, it can be divided into three types, respectively strongly basic (including quaternary amino group), weakly basic (including tertiary and secondary amino group) and mediumly basic (containing both strong basic group and weak basic group).
When they exchange with ions in solution, the reaction formula is the following:
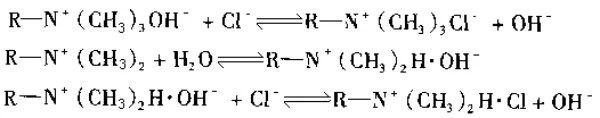
For more information about the specific types of ion exchange chromatography resins, stay tuned on our next article on Ion Exchange Chromatography.















