
What is Ion Exchange?
Ion exchange is a chemical separation technique that emerges after separation methods such as distillation, extraction, and absorption.
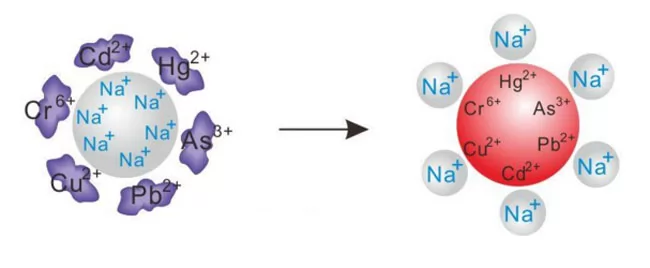
The ion exchange is a technique for separating using an exchangeable group in an ion exchanger and a difference in ion exchange capacity between various ions in a solution, and is a method of solid-liquid separation.
Ion exchange generally refers to a reversible solid-liquid chemical reaction in which the solid is an ion exchange resin. The ion exchange resin consists of an inert backbone, a fixed chemical group and a mobile ion (also called a swap ion or a counter ion): a cation exchange resin R-SO3M, where R is an inert skeleton and SO3 is a fixed group. M is a living ion or an exchangeable ion, and SO3M is a reactive group or a functional group.
Characteristics of ion exchange technology
1. The ion exchange operation belongs to the liquid-solid heterogeneous diffusion mass transfer process. The solution to be treated is generally an aqueous solution, and the multiphase operation makes separation easy.
2. Ion exchange can be thought of as the process of ion exchange reaction between the separated components in the solution and the exchangeable ions in the ion exchange resin. The selectivity is high, and the ion exchange reaction is quantitatively performed, that is, the mole of the ions adsorbed and released by the ion exchange resin is equal.
3. After the ion exchange resin is used, its performance gradually disappears, and it needs to be regenerated by acid or alkali to be used again.
4. The ion exchange technology has advantages of high concentration and enrichment, and the operation is convenient and the effect is outstanding.
Application of ion exchange technology
Ion exchange is used for extraction, separation, concentration and refining.
1. Water treatment (softening of water, demineralization of water, preparation of condensed water and ultra-pure water)
2. Food, beverage, sugar (decolorization, demineralization, pesticide removal, HMF removal, acid removal)
3. Biochemical extraction (separation and recovery of natural biological substances, separation and recovery of fermentation products, application of pharmaceutical industry)
4. Wastewater Treatment (treatment of radioactive wastewater, treatment of other industrial hazardous waste water)
5. Hydrometallurgy (Gallium, lithium, precious metal extraction)














